The science of road salt
With an impactful winter storm possible next week, preparation might be on your mind. Most people are familiar with roadways and side-walks looking like this each winter. But just how does this salt help clear your roadways?
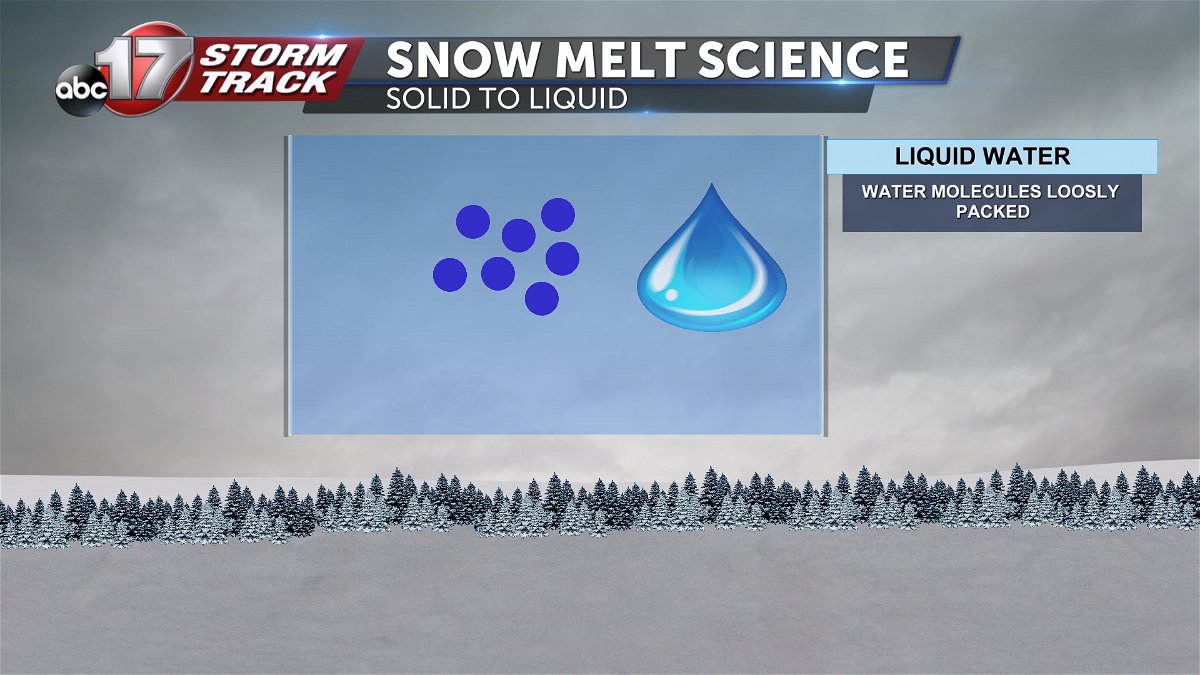
Water in it's liquid form consist of loosely packed molecules. This allows for it to flow easily and fit the shape of the container it is in.
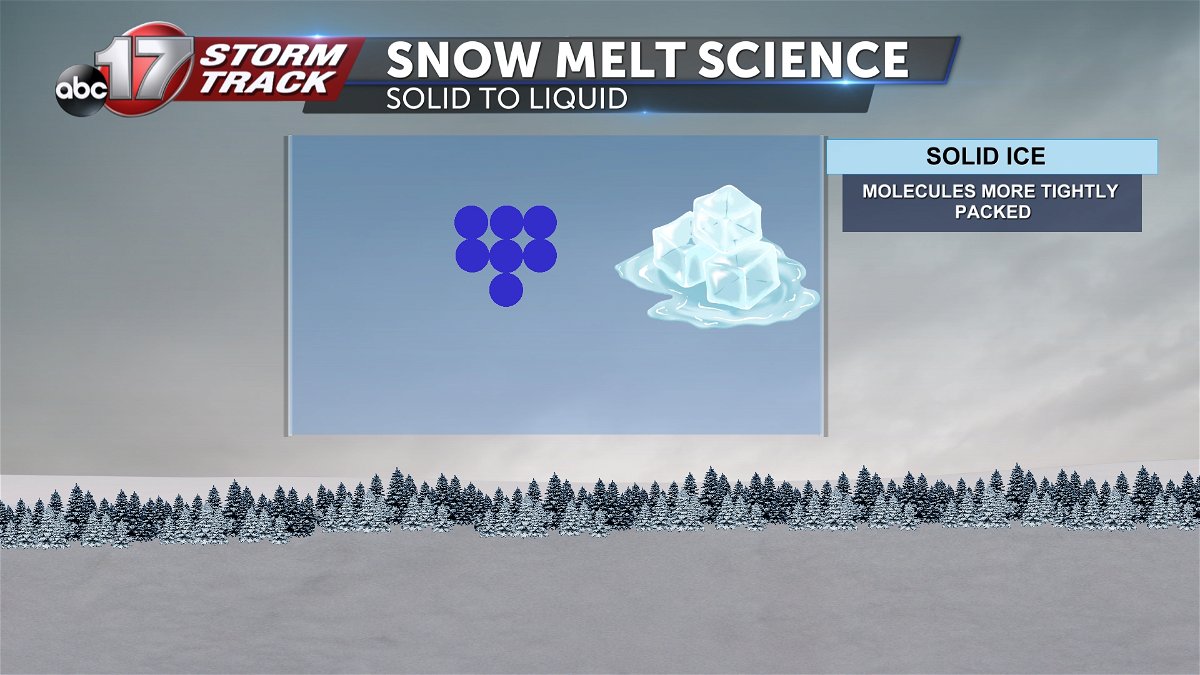
Whenever water reaches the freezing point of 32 degrees or below, molecules are then arranged more tightly compacted giving it the solid shape. Water expands when it freezes because of how it crystallizes, despite the molecules becoming more tightly packed.
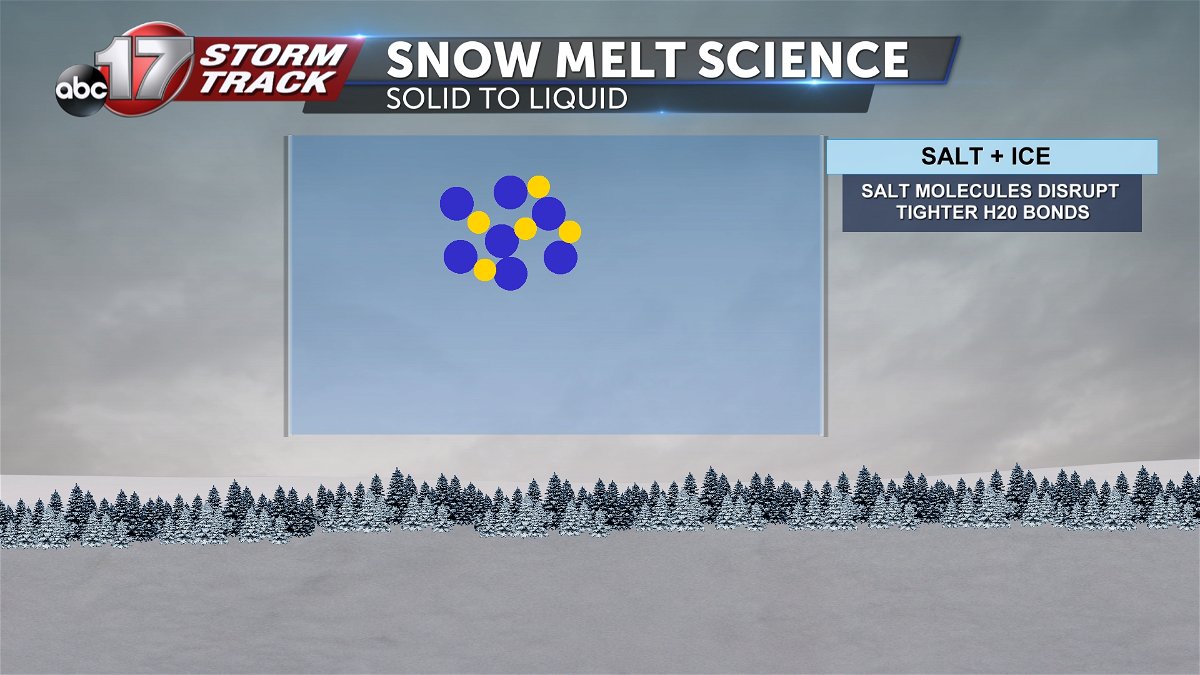
Salt does not necessarily melt ice or snow with increased heat. It merely brings the freezing point of water to lower temperatures. At the molecular level, the salt molecules get in the way of the bonding h20 molecules reducing water back to its liquid state.

Understanding which ingredients are in your salt are also important as the temperature cools. The compound make-up determines the salts effectiveness in well-below freezing temperatures.
Using a mixture of water also allows you to get more bang for your buck as the salt brine uses less salt to melt more of an area. The drawback is that the freezing temperatures increases so be careful in cooler temperatures. it is also important to not overuse salt as it has negative impacts on the environment and to make sure you check for the pet safety seal so your furry friend stays healthy.
